- Validation of a New Sham Acupuncture Needle for Double-Blind Trials: A Study Protocol
-
Sung Min Lim
-
Perspect Integr Med. 2024;3(1):57-60. Published online February 22, 2024
-
DOI: https://doi.org/10.56986/pim.2024.02.008
-
-
 Abstract Abstract
 PDF PDF
- Background
To establish efficacy in acupuncture treatment, rigorous randomized controlled trials (RCT) are needed. Non-invasive sham acupuncture needles are an effective tool for practitioner/participant blinding. This study presents a protocol for the validation of a newly developed sham acupuncture needle.
Methods
A double-blind RCT will be conducted on 66 healthy adults who will be randomly assigned (using computer-generated random numbers) to either the verum (n = 33) or sham (n = 33) acupuncture needle group. The needles will be inserted at 2 acupuncture points: LI4 (upper limb) and ST36 (lower limb). The primary outcome measure is the practitioner/participants belief that they received verum or sham acupuncture. The secondary outcome measures are participant-rated sensations (penetration, pain, and de qi). Adverse events will be recorded with detailed explanations, categorizing occurrences according to related or unrelated to acupuncture. As the newly developed sham acupuncture has not been studied before, an exploratory approach has been adopted. Descriptive statistics, t test, and χ² test will be applied appropriately.
Results
This study is intended to provide a protocol for the validation of a sham acupuncture needle by using a double-blind RCT setting, and the results will hopefully contribute to the standardization of the needles used for sham acupuncture. The outcomes aim to determine the reliability of practitioner/participant blinding, participant experience of sensations, and lay groundwork for a standardized control group for clinical trials in the future. The newly developed non-invasive sham acupuncture needle may reduce bias and improve reliability in the size effect of acupuncture treatment.
- Systematic Review Protocol for Sham Acupuncture Validation Research
-
Sung Min Lim
-
Perspect Integr Med. 2023;2(2):131-133. Published online June 23, 2023
-
DOI: https://doi.org/10.56986/pim.2023.06.008
-
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
 - Background
Streitberger and Park sham needles have been developed and used as non-penetrating sham acupuncture needles that can be blinded in randomized controlled clinical trials assessing the efficacy of acupuncture. Ideal sham acupuncture should not be distinguishable from an actual acupuncture treatment provided to the experimental group to ensure patient blinding; additionally, it should not have any physiological or biological effect. Providing evidence for such sophisticated sham acupuncture devices is critical, as control settings in clinical studies are based on research verifying their validity.
Methods
Three core electronic databases - PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials - will be used to search for validity verification studies of sham acupuncture devices. Clinical studies that verify the validity of non-penetrating sham acupuncture devices will be included in the review.
Results
The study design, participant information, experimental and control groups, study population’s experience with acupuncture, outcome variables, and results of studies that verify the validity of sham acupuncture devices will be systematically reviewed.
Conclusion
This systematic review of validity verification studies of sham acupuncture devices is expected to help the development of more sophisticated sham acupuncture, as well as the design of studies verifying its validity in the future.
- Trends in the Development of Sham Acupuncture-Related Technologies Focused on Patents Applied for in South Korea
-
Sung Min Lim
-
Perspect Integr Med. 2023;2(1):36-41. Published online February 21, 2023
-
DOI: https://doi.org/10.56986/pim.2023.02.005
-
-
1,650
View
-
20
Download
-
2
Citations
-
 Graphical Abstract Graphical Abstract
 Abstract Abstract
 PDF PDF
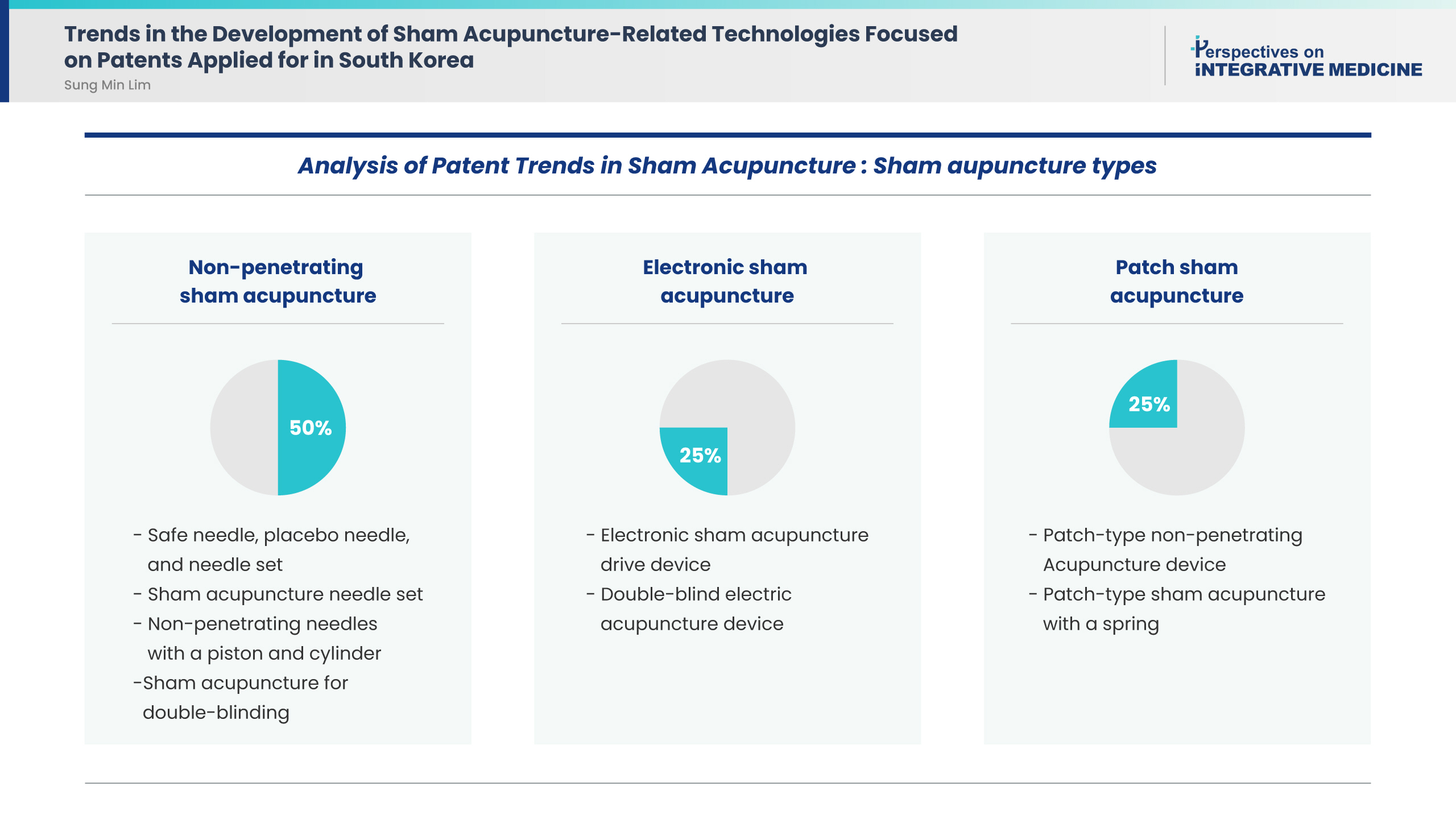 - Background
This study aimed to analyze the trends in Korean patents for sham acupuncture.
Methods
The electronic database of the Korea Intellectual Property Rights Information Service was searched for Korean patents for sham acupuncture from inception till September 2020. Patents, which were not related to sham acupuncture, were excluded. The applicant, application date, International Patent Classification, and technological content of sham acupuncture were analyzed.
Results
This study included eight patents. Application analysis identified the following sham acupuncture types: four (50%), two (25%), and two (25%) patents were for non-penetrating sham acupuncture, electronic sham acupuncture, and patch sham acupuncture, respectively. All patents aimed to use sham acupuncture as a control for rigorous double-blind clinical trials to verify the efficacy of real acupuncture treatment.
Conclusion
The present findings suggest that technological advances were focused on developing various types of sham acupuncture methods for double-blind studies. Further large-scale studies using rigorous designs are needed to investigate new sham acupuncture applications.
-
Citations
Citations to this article as recorded by  - Validation of a New Sham Acupuncture Needle for Double-Blind Trials: A Study Protocol
Sung Min Lim
Perspectives on Integrative Medicine.2024; 3(1): 57. CrossRef - Systematic Review Protocol for Sham Acupuncture Validation Research
Sung Min Lim
Perspectives on Integrative Medicine.2023; 2(2): 131. CrossRef
|